Home / Personal iPS Cell Banking Service

iPSCs are the only one type of stem cells which can be made from anyone and can be changed to any type of cells in our body. If the iPSCs are stored when you are healthy, the banked iPSCs can help you overcome different illness and injuries when needed.
My Peace’s personalized iPS cell products are created at a licensed facility approved by the Minister of Health, Labor, and Welfare in Japan. This facility also complies with the standards of the U.S. FDA. As clinical trials and studies for various transplant therapies using iPS cells are currently underway, My Peace’s iPS cells are available for various usage once autologous transplants using iPS cells is commercialized.

I Peace manufactures iPS cells for clinical trials for major pharmaceutical agencies all around the world. Our experience, technologies, and advanced quality control all contribute to ensuring the conscientious creation of iPS cells for personalized medical use. I Peace is pleased to provide iPS cells based on our world-class quality assurance.
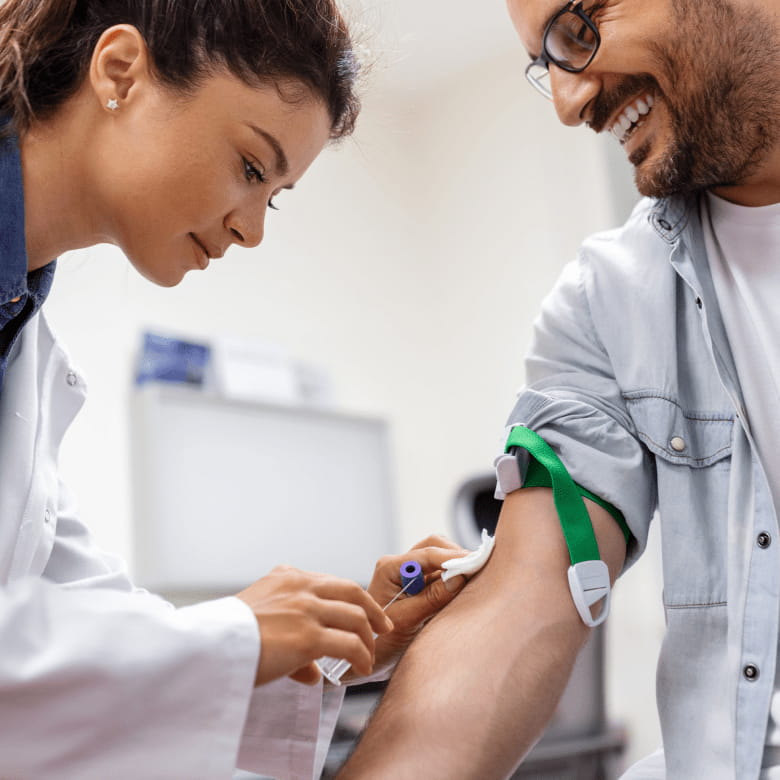
The best cell collection method for clinical-grade cell production

I Peace’s personal iPS cells are manufactured and stored in approved facilities. All processes comply with the official FDA guidelines in the USA and Japan. It, in fact, fulfilled the highest global quality standard. The manufacturing facility has been approved by the Kinki Bureau of the Ministry of Health, Labor, and Welfare of Japan. This approval is only granted after a series of strict inspections at the frontline of the manufacturing process. In addition, the facility has been inspected and approved based on the standard FDA guidelines (USA)



Reduce future risks
Cell therapy manufacturing is time consuming. It takes a certain amount of time to generate iPS cells from a customer’s blood, evaluate their quality, and produce the type of cells needed for transplantation. Depending on the type of injury or disease, time could be a fatal constraint. Our life could be much more secured if we possess our own iPS cells preserved in a safe manner.
Avoid immune rejection
The human body has an immune system rejecting cells from another human body. Therefore, cells transplanted from someone else would be unfortunately attacked by our immune system and got killed. One of the keys to success in cell transplantation is, therefore, whether the transplanted cells can settle in the body without being rejected by the immune system. Transplanting cells made from your cells would avoid immune rejection and maximize the effectiveness of cell transplantation therapy.

Contributing to the acceleration of iPS cell research
Customers who agree to cooperate in the research (cooperation in the research is voluntary) will also help to put iPS cells in the research, thereby promote the development of treatments for intractable diseases and in turn contribute to save lives in the future, and release those suffer from terminal diseases.
Contributing to the acceleration of iPSC-derived cell therapy and drug discovery using iPS cells *1
Treatment with your own iPSC-derived cells *2
Contributing to the acceleration of iPSC-derived cell therapy and drug discovery using iPS cells *1
Treatment with your own iPSC-derived cells *2

